Gently shake the contents of the bag while holding the bag over the plate. Calcium chloride anhydrous CaCl2.

Title Chemistry In A Ziploc Bag Pages 1 5 Flip Pdf Download Fliphtml5
A ziploc bag make look like it wont let anything in or out but it actually has tiny holes that will.
. Add 2 drops of the 02 phenol red solution to the water. To observe the chemical reactions of substances mixed together. Goggles must be worn at all times in the laboratory.
The contents if the vial MUST NOT SPILL. Place ½ spoonful of calcium chloride into the bag. Open the bag while a VSVS member goes around and adds a teaspoon of calcium chloride.
Up to 24 cash back Put one scoop full of baking soda in a zip lock bag Put two scoops of calcium chloride in the same bag. Give each pair one ziploc bag containing baking soda one 1 oz cup containing 15 mL of phenol red solution and one plate. Chemistry in a Ziploc Bag.
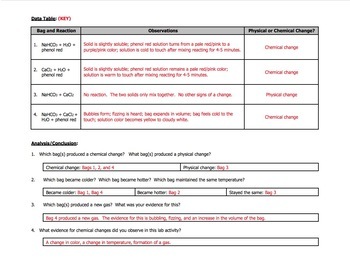
When the anhydrous calcium chloride the mixture becomes warm at first because anhydrous calcium chloride gives off heat when it dissolves in water. Chemistry in a Ziploc Bag. Chemistry in a Ziploc Bag.
The bag feels cold because baking soda absorbs heat when it dissolves in water. Carefully put the vial into the baggie without spilling the indicator. Open the bag while a VSVS member goes around and adds a teaspoon of calcium chloride.
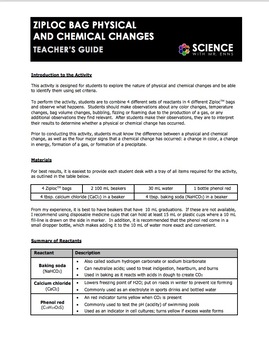
Chemistry in a Ziploc Bag In this experiment students will have the opportunity to observe some chemicals and the chemical reactions that occur when these chemicals are combined in a closed container. Add 5 mL of Phenol Red to the film canister. Open the bag while a VSVS member goes around and adds a teaspoon of calcium chloride.
Place the open plastic bag on the table open edge facing up. 4 Ziploc bags. Warn them that the ziploc bag is going to fill up with air.
Mix the two powders together in the bag. -3 Ziploc bags -3 small sandwich baggies -sodium bicarbonate baking soda NaHCO3 -calcium chloride CaCl2 Procedure. VERY CAREFULLY - lower the film canister containing the 30 mL of liquid upright into the bag.
Calcium chloride is hygroscopic and will pick up wate r from the air. 30 mL Indicator solution phenol red 30 mL red cabbage juice Procedure 1. Chemistry questions and answers.
Hold the bag upright over the plate. Measure 10 ml-a of bromothymol blue into a plastic vial. Place 1 level spoonful of Calcium chloride in the other corner of the bag.
30 mL water. When three substances are mixed in a sealed clear plastic bag chemical reactions occur that causes. Phenol red solution 2 plastic teaspoons 2 10-mL containers vials 6 Zip-Loc bags quart size.
The phenol red solution then turns yellow which indicates an acidic solution. Answer to Chemistry in a Ziploc BagPre Lab Questions--plz. Add the phenol red solution including water to the bag and seal the bag.
Chemistry in a Ziploc BagPre Lab Questions--plz answer in detail. Place 2 level teaspoons of calcium chloride CaCl 2 and 1 level teaspoon of sodium bicarbonate NaHCO 3 in a quart sized Ziplock bag and record observations after each addition. Pour 10 mL of cabbage juice into a small vial.
Measure 10 mL of the phenol red solution in the marked plastic vial and pour it into the small plastic bag. In the event one bag leaks or explodes use paper towels to clean up any mess The VSVS team should collect all Ziploc bags and used cups and put them in the trash bag Make sure the Ziploc bags with the reaction mixture is sealed before you put them in the trash bag Put everything else in the kit box along with the. Place 1 level spoonful of Calcium chloride in the other corner of the bag.
Sodium hydrogencarbonate to a Ziploc bag. Add the phenol red solution including water to the bag and seal the bag. Into a one-gallon Ziploc bag place 2 cups of ice.
Mini-lesson Vanderbilt Student Volunteers for Science Spring 2014. For this experiment students work in pairs. Add one spoonful of sodium bicarbonate baking soda to the bag.
And i m very much worriedquestions a The equations for the dissolution of Calcium Chlorideand sodium. Introduction Tell students this lesson involves a chemical reaction that produces a gas. All thnks in advanee i have a labdis week 1st lab of my sem.
Hold the bag upright over the plate. If any bromothymol blue is dripping down the outside of the vial use paper towel to wipe this off VERY CAREFULLY place the vial UPRIGHT in the bag. Click to see full answer Thereof do Paper bags have chemicals.
Place one spoonful of sodium bicarbonate in a Ziploc bag. Using the pipette add 5mL of phenol red into the small glass vial 4. Hashim Period 7 8 101006.
Up to 24 cash back. Assure students that the chemicals are safe. Do not allow them to touch.
A question is investigated through careful and accurate recordings of observations. Up to 24 cash back Place 1 spoonful of baking soda into the bag. Add 25 mL of water to the film canister.
Add 2 drops of the 02 phenol red solution to the water. Measure 1 teaspoon of calcium chloride and add it into the same bag 3. Lay the plastic bag on the lab counter.
Place 1 level spoonful of Sodium bicarbonate in one corner of the bag. 35mm film canister top optional Small paper cup. For common commercial grades of medium- and high-density polyethylene Ziploc the melting point is typically in the range 120 to 130 C 250 to 265 F.
Place 1 spoon of NaHCO3 baking soda in the left hand corner of the bag and 1 spoon of CaCl2 calcium chloride in the right corner of the bag. Hold the bag upright over the plate. While plastics can be high tech advanced materials.
Sodium hydrogencarbonate Baking soda. Zip the bag closed and shake it to observe for any evidence of a chemical change. Chemistry questions and answers.
Immediately work the air out of the bag and seal it. Tell students that knowledge in science is obtained through the scientific method. Give each pair one ziploc bag containing baking soda one 1 oz cup containing 15 mL of phenol red solution and one plate.
Add the phenol red solution including water to the bag and seal the bag. 1-gallon bromothymol blue indicator 10-ml graduated cylinders one per lab group teaspoons 1 to 2 per lab group 3 pounds calcium chloride CaCl 2 from chemical supply house or from a store selling this type of road salt or laundry aid 1-12 pounds sodium bicarbonate NaHCO 3 baking soda Activities. Vanderbilt Student Volunteers for Science Fall 2013 8 th grade.
See answer 1 Best Answer. Baking soda sodium bicarbonate NaHCO3. Measure ½ a teaspoon of sodium bicarbonate and place it into the plastic bag 2.
Measure one spoonful of calcium chloride and place it into the ziplock bag.

Ziplock Chemistry Thermodynamics
Lab Ziploc Bag Physical And Chemical Changes By Science With Mr Enns
Lab Ziploc Bag Physical And Chemical Changes By Science With Mr Enns

Title Chemistry In A Ziploc Bag Pages 1 5 Flip Pdf Download Fliphtml5

Lesson Plan Bundle Ionic And Covalent Bonding Distance Learning Covalent Bonding Teaching Chemistry Chemistry Worksheets


0 comments
Post a Comment